Epidemiological analysis for Hereditary Angioedema disease (EHA Study)
EHA is an international, multicenter, epidemiological observational study investigating the prevalence of Hereditary Angioedema (HAE) disease among participants with recurrent episodes of abdominal pain of no obvious etiology.
Summary
Overview
The Epidemiological analysis for Hereditary Angioedema (EHA) is an international observational study. The main goal of the study is to investigate the prevalence of Hereditary Angioedema (HAE) among participants with recurrent episodes of abdominal pain of no obvious etiology. Moreover, the study aims to establish HAE biomarker(s) that may potentially facilitate earlier diagnosis and treatment personalization in the future.
Participant Benefits
Participants may receive a definite diagnosis of HAE type 1/2. This may also have a direct impact on the family of the diagnosed subjects due to the dominant inheritance nature of the disease.
Clinical Trials
Study access on CentoPortal® for participating physicians
Study Rationale & Design
Study Rationale
HAE is an autosomal dominant inherited disorder that is often under- or misdiagnosed. Recurrent abdominal pain attacks, which are sometimes the only manifestation of the disease, may be mistaken for other conditions, such as psychiatric disorders or appendicitis – resulting in unnecessary medical and surgical procedures.
The disease is usually not diagnosed until late adolescence, and undiagnosed individuals may have frequent abdominal pain episodes that may impair their quality of life. The EHA study will help in providing an early diagnosis of such individuals, and thus update the prevalence of HAE. Moreover, the biochemical analyses carried out on HAE-positive samples will further support the development of new diagnostic tools for HAE.
Study Design
Participants fulfilling the eligibility criteria may be enrolled in the Study. All participants will have a single blood sample drawn (around 1ml). This will be applied to a CentoCard®, which will be sent to CENTOGENE and analyzed at CENTOGENE’s specialized laboratories for HAE. The diagnostic workflow is a two-step approach: In the first step, the complement proteins are quantified by tandem mass spectrometry, and in the second step, the results are confirmed by genetic analyses. The biochemical results are thus genetically confirmed via Next Generation Sequencing of the SERPING1 gene, and when necessary, Multiplex Ligation-dependent Probe Amplification (MLPA) is performed to identify large deletions or duplications.
CENTOGENE will contact the study doctor to inform them about the test results. Only participants diagnosed with HAE will be informed by their study doctor. If the subject is not contacted by the study site within one month, the results can be considered negative.
Information About the Study
Design: International, multicenter, epidemiological, observational study
Study population: Individuals with previous episodes of abdominal pain attacks of no obvious etiology
Number of participants: 5,000 participants
First participant in: September 2018
Last participant in: June 2022
Inclusion period: 48 months
Objectives:
Primary – To investigate the prevalence of HAE in individuals with previous episodes of abdominal pain of no obvious etiology
Secondary – To establish a biomarker in the HAE-positive cohort
Find out how you can participate: ClinicalTrials.gov | Recruitment is closed.
Inclusion Criteria
| Inclusion Criteria |
|---|
| Informed consent is obtained from the participant or parent/legal guardian |
| Participant has experienced previous episodes of abdominal pain of no obvious etiology |
| Participants aged between 2 to 60 years old |
Geographic Scope
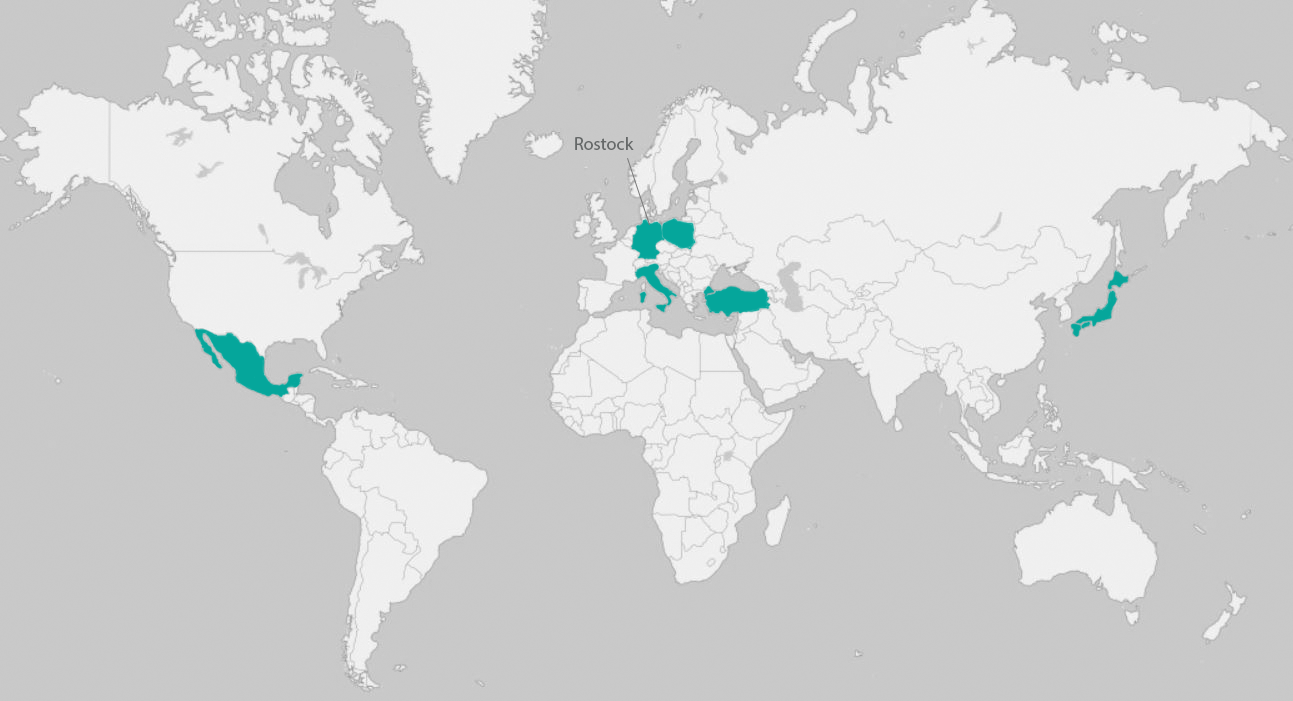
The EHA study is being conducted in Mexico, Germany, Italy, Turkey, Poland, and Japan.
HAE Disease Facts
- A hereditary disorder characterized by bradykinin-mediated angioedema
- An autosomal dominant disease caused primarily by mutations in SERPING1 gene, coding for the plasma C1 esterase inhibitor (C1-INH) protein
- Published prevalence varies from 1:10,000 to 1:50,000
- 93% of HAE patients suffer from recurrent abdominal pain due to gastrointestinal edemas, which might be the only manifestation of disease
- Individuals with gastrointestinal symptoms are rarely suspected of having HAE and may face misdiagnosis for an average of eight years
Resources
Quick Links
Contact EHA Study
For more information please contact
Dr. Anika Knaust, Clinical Study Researcher
CENTOGENE GmbH
Am Strande 7
18055 Rostock
Germany
Email: Anika.Knaust@centogene.com
Limor Oren, Clinical Research Associate
CENTOGENE GmbH
Am Strande 7
18055 Rostock
Germany
Email: limor.oren@centogene.com
