Rostock International Parkinson's Disease Study (ROPAD)
ROPAD is an international, multicenter, epidemiological, observational study with the goal to investigate the genetic background of Parkinson’s patients.
Summary and Rationale
Overview
The Rostock International Parkinson's Disease Study (ROPAD) is a global observational study focusing on the role of genetics in Parkinson's disease (PD). The major goal of the study is to characterize the genetics of PD to establish a better understanding of the disease progression, diagnosis, and treatment. Throughout this study, up to 25,000 PD participants from around the world will be tested.
Clinical Trials
Study access on CentoPortal® for participating physicians
ROPAD Study Rational and Screening Strategy
PD can be caused by several factors, including environmental factors, genetics, and aging. In recent years, a growing number of PD related genes have been described – including LRRK2 and GBA. Thus, the identification of new genes and mutations associated with PD will not only improve our understanding of the underlying molecular mechanisms, but may also lead to the development of new drugs and treatments.
Participants with LRRK2 mutations that meet the eligibility criteria may be offered participation in future clinical studies with our partners who are developing therapies for the treatment of neurodegenerative and other human diseases.
Information About the Study
Design: International, epidemiological, observational, non-interventional study
Study population: PD patients
Number of participants: Up to 25,000 participants
First participant in: April 2019
Last participant in: December 2025
Objectives: To investigate the prevalence of genetic etiologies of PD; To identify LRRK2-positive PD participants from the primary strata; Establishment of a biomarker in the LRRK2-positive cohort
Find out how you can participate: ClinicalTrials.gov
Geographic Scope
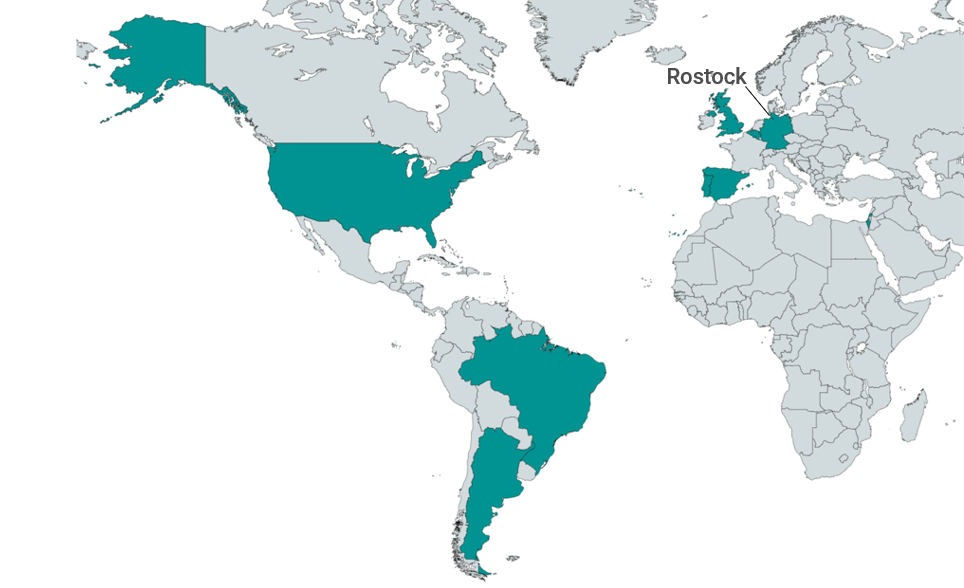
ROPAD is a global study currently being conducted in the United States, UK, Argentina, Brazil, Germany, Italy, Belgium, Portugal, Spain, and Israel. Participating countries and the geographic distribution facilitate a broad genetic and ethnic background that mirrors the global population.
Parkinson’s Disease Facts
- One of the most common neurodegenerative disorders worldwide: Approximately 1% of individuals over 60 years are affected
- Likelihood of developing PD increases with age and is more frequent in males
- Both genetic and environmental factors may contribute to PD
- Characterized by pathological toxic protein deposits in neurons, leading to a deficiency of neurotransmitters such as dopamine
- Clinical symptoms include tremors, muscle rigidity, postural instability, and gait abnormalities
Study Design and Information for Participants:
Who can participate in the ROPAD Study?
The participation in the study is available for individuals between 30 to 80 years of age and who fulfill the following criteria:
- Informed consent is provided.
- Clinically diagnosed with Parkinson's disease within the last 5 years (≤ 5 years)
What should I expect from participating in the ROPAD Study?
As a participant in the ROPAD Study, you will be asked to provide a signed consent form, a small blood sample, and a short clinical history, as well as to perform a neurological exam.
The blood sample will be sent and analyzed in CENTOGENE’s laboratory for mutations that may be relevant to PD.
If you have agreed to being informed about the genetic testing results, your doctor will share the medical report with you.
After receiving your genetic test results, your doctor can provide further consultation. During counseling, you will have the opportunity to gain a deeper understanding of your medical results.
Working Together
CENTOGENE is working with pharmaceutical partners who develop investigational therapies for the treatment of neurodegenerative diseases, as well as others. Participants testing positive for certain mutations and that meet eligibility criteria may be offered participation in clinical studies.
Contact ROPAD Study
For more information please contact
Project Manager
CENTOGENE GmbH
Am Strande 7
18055 Rostock
Germany
Email: ropad@centogene.com
