CentoNIPT® – Non-Invasive Prenatal Testing at CENTOGENE
Get your results within 5 business days. CentoNIPT offers genetic, non-invasive prenatal testing (NIPT) to screen for the most common fetal chromosomal abnormalities. Our test combines the latest next generation sequencing technology with expert medical reporting.
*Note: CentoNIPT is unavailable in the US.
With CentoNIPT CENTOGENE Now Offers Non-Invasive Prenatal Testing That Provides a Fast and Accurate Screen for the Most Common Prenatal Chromosomal Abnormalities.*
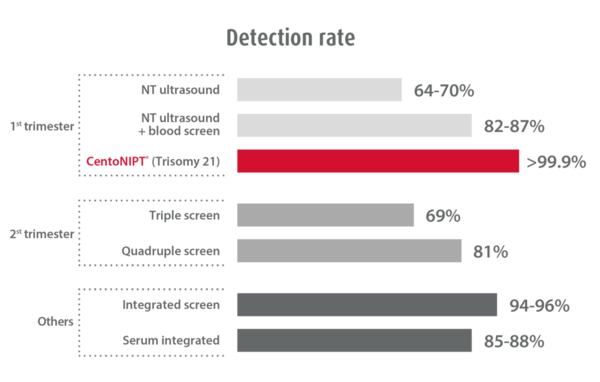
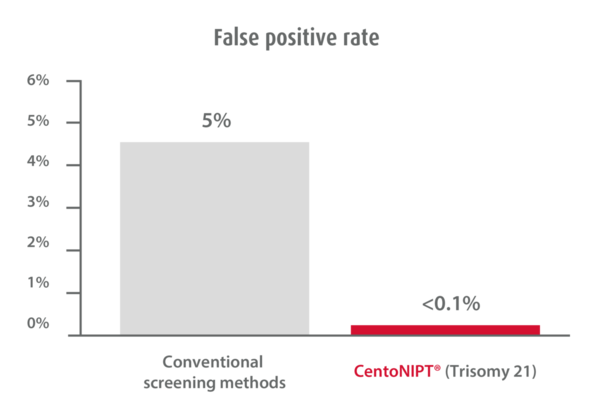
CentoNIPT is performed on a single maternal blood sample and combines the latest next generation sequencing technology with the highest quality medical reporting. It provides unparalleled accuracy and detection compared to other non-invasive testing methods – ultrasonography or nuchal translucency testing.
Our medical expertise is ideally suited to provide you and your patients with reliable, well supported result interpretation.
Why Choose CentoNIPT?
100% safe
Only 9ml of maternal blood required
as early as possible
Test performed from the 10th gestational week
Fast & RELIABLE results
Highly accurate results available within 5 business days
Easy HAndling
CentoNIPT® kit free of charge. Return shipment via phone call
Illumina VeriSeq™ NIPT Solution v2
Expertise You Can Trust
Conventional prenatal testing for fetal chromosomal abnormality involves either chorionic villus sampling or amniocentesis. These procedures are highly invasive and carry an elevated risk of miscarriage, but despite this have become standard practice worldwide due to their high levels of accuracy and the range of abnormalities they can detect.
Approximately 1% of All Babies Will Be Born With a Chromosomal Abnormality
- Fetal chromosomal abnormalities are causing physical disability and/or mental retardation
- 70% of syndromic congenital abnormalities are contributed by Trisomy T21, T18 or T13 and 10% by Turner syndrome (Monosomy X)
- The risk of trisomy increases significantly with maternal age
Trisomies
| Trisomies | Sensitivity | Specificity |
|---|---|---|
| Trisomy 21 (Down syndrome) | >99.9% | 99.9% |
| Trisomy 18 (Edwards syndrome) | >99.9% | 99.9% |
| Trisomy 13 (Patau syndrome) | >99.9% | 99.9% |
Sex Chromosome Aneuploidies & Fetal Gender
| Sex Chromosome Aneuploidies | Concordance with cytogenetic results |
|---|---|
| XX | 100.0 % |
| XY | 100.0 % |
| X0 (Turner syndrome) | 90.5 % |
| XXX (Triple X syndrome) | 100.0 % |
| XXY (Klinefelter syndrome) | 100.0 % |
| XYY (Jacobs syndrome) | 91.7 % |
CentoNIPT Reporting Information
CentoNIPT is only designed to analyze chromosome aneuploidies of the fetus after 10 weeks of gestation. Reported are overrepresentations of chromosomes 21, 18 and 13, as well as the sex chromosome aneuploidies XO, XXX, XXY and XYY. This screening test does not test for aneuploidies in other chromosomes not mentioned above and can therefore not exclude abnormalities in these.
Chromosome aneuploidies for a twin gestation can in general be detected by this test. However, the test cannot be attributed to individual twin fetuses because in twin gestations sensitivity and specificity for detection of aneuploidies are limited. In case of twin pregnancy and detection of only one Y chromosome by the test, the fetal gender of each individual twin cannot be determined by the test.
CENTOGENE Reports NIPT Results As Follows:
Positive result – if CentoNIPT identifies an aneuploidy (chromosome 21, 18, 13 or gonosomal chromosomes), CENTOGENE reports presence of aneuploidy and provides data on fetal DNA fraction in the sample of mother´s peripheral blood (in percentages) and the data on fetal gender if requested. We urgently recommend to the referring physician or the genetic counselor to determine and decide if further invasive testing and subsequent analysis is needed.
Negative result – if NIPT did not indicate a trisomy of chromosome 13, 18, or 21 or gonosomal abnormalities, CENTOGENE reports a negative result together with the data on fetal DNA fraction and the data on fetal gender if requested. A negative result cannot entirely exclude the possibility of fetoplacental mosaicism. Thus, CENTOGENE is giving negative results of NIPT together with the suggestion: if the fetus shows abnormalities on ultrasound investigation, or if a family history of fetal abnormalities or other genetic disorders exists, we urgently recommend to the referring physician or the genetic counselor to determine and decide if further invasive testing and subsequent analysis is needed.
In the very unlikely case of inconclusive results of the non-invasive prenatal testing due to the limitation of the system, we recommend further follow-up of the fetal growth using ultrasound as well as 2nd trimester screens. In case of any abnormalities observed on the ultrasound examination or if there is a positive family history of fetal abnormalities or other genetic disorders, we highly suggest invasive testing and subsequent analysis.
Notation
Sample Preparation and analysis software are CE-IVD marked
Noninvasive prenatal testing (NIPT) based on cell-free DNA analysis from maternal blood is a screening test; it is not diagnostic. Test results must not be used as the sole basis for diagnosis. Further confirmatory testing is necessary prior to making any irreversible pregnancy decision.

Resources
Quick Links
Downloads

Request Form – CentoNIPT
Non-invasive prenatal testing

CentoNIPT Brochure
Expertise you can trust

CentoNIPT – Sample Report, Negative
NEGATIVE RESULT – No aneuploidy identified
Scientific Publications
Proof-of-Principle Rapid Noninvasive Prenatal Diagnosis of Autosomal Recessive Founder Mutations
Proof-of-principle rapid noninvasive prenatal diagnosis of autosomal recessive founder mutations. Ten parental alleles in eight unrelated fetuses were diagnosed successfully based on the noninvasive […]
Frequently Asked Questions Non Invasive Prenatal Testing
This analysis is restricted to fetal cells from the placenta and not from the fetus itself. Fetoplacental mosaicism, a different chromosomal setup for placenta and fetus, is very rare but cannot be ruled out. Although sensitivity and specificity of NIPT are indeed high, it is necessary to confirm such results by additional method(s), such as echosonographic findings, maternal serum analysis; or perhaps amniocentesis for confirmation of a positive result.
Yes. The fetal fraction is included in the result.
Yes. However, the gender information will be provided only after 12 weeks of gestation, as regulated by German law, as the test is performed in Germany.
We report aneuploidy, the occurrence of additional chromosomes in the fetus. However, this analysis is restricted to those chromosomes where a living fetus could be born with abnormalities. These chromosomes include chromosome 13, 18, 21 and the sex chromosomes.
The analysis only reports if an additional chromosome has been identified
YES – the data indicate an extra copy of one chromosome. We urgently recommend to the referring physician or the genetic counselor to determine and decide if further invasive testing and subsequent analysis is needed.
NO – the data do not indicate any extra copy of the aforementioned chromosomes.
CentoNIPT® is based on the in vitro diagnostic test Illumina VeriSeq™ NIPT Solution v2. This noninvasive IVD test utilizes whole-genome sequencing on cell-fetal DNA (cfDNA) fragments derived from maternal peripheral whole blood samples. After whole genome sequencing and bioinformatics analysis, chromosome read numbers and fetal fraction are combined and thus translated into chromosome ploidy. Finally, the comparison of the tested chromosomes by this test (21, 18, 13, X and Y) with reference chromosomes enables the identification of aneuploidies, which are then reported.
Related Webinars
生殖健康与產前遺傳学檢測
研討會中,我們論述了 CENTOGENE 產前篩查選項,以及產前檢測如何在孕週期時發揮關鍵作用,快速尋找答案。
Reproductive Health and Prenatal Genetic Testing
Throughout the CentoTalk, Clarice Loh provides a detailed overview of CENTOGENE’s prenatal screening options and how prenatal testing plays an essential role in facilitating early answers when […]
What You Need to Know about Carrier Screening
Carrier screening is a predictive genetic testing, designed for future parents. Would you like to learn more and understand it better? Watch our recorded webinar about CentoScreen®: "What you need to […]
Get in Touch With Our Customer Support
Our consultation service is available in several languages.
+49 (0) 381 80 113 - 416
Mon. – Fri. 7 a.m. – 6:30 p.m. CET
Sat. 8 a.m. – 12 p.m. CET